Fuel Cell Electrochemistry Pdf
The equilibrium cell potential Eeq where jL is the limiting current density. 3 WS2002 3 Electrochemistry Electricity can be generated by burning a fuel using the heat to run a heat engine and using the heat engine to run a generator The efficiency of this process is limited by the second law of thermodynamics 33 is typical for a modern plant Fuel cells electrochemical cells are not limited by the second law Efficiency can be much higher.

Electrochemistry Notes And Galvanic Cell Notes Galvanic Cell Chemistry Notes Redox Reactions
Almost every car producer has a fuel cell car prototype but still there are several obstacles to overcome before mass.
. A the area of the cell. Overall chemical reaction is the same Reactant is transported as an ion O2-in example above. Electrolyte fuel cell products can be found today mainly in the lower power range and many more are close to commercialisation.
¾In low-temperature fuel cells all the fuel must be converted to hydrogen prior to entering the fuel cell. At 25 C 298K and atmospheric pressure it is equal to 123 V. Pollet FRSC HySA Systems University of the Western Cape South Africa.
J0 the exchange current of a fuel cell in terms of temperature and gas compositions is density. Klett Parsons Corporation Reading PA 19607 Under Contract No. The reactions carried out electrochemically can be energy efficient and less polluting.
One electrode called the anode facilitates electrochemical oxidation of fuel while the other called the cathode promotes. Download or Read online Electrochemical Fuel Cells full HQ books. Available in PDF ePub and Kindle.
Fuel Cell Introduction and Analysispdf - CHBE 477577. It contains a variety of questions from previous years AIIMS NEET and. FUEL CELL FUEL CELL FUEL CELL Fourth Edition November 1998 Fuel Cell Handbook November 1998 DOEFETC-991076 by JH.
Electrochemistry is the study of the relationship between chemical change and electrical work. Electrochemistry is a branch of chemistry which addresses the interrelation of chemic al. Most of the fuels used are hydrocarbons so directly or indirectly natural gas- oil- or coal-based.
It is examined via the use of electrochemical cells which are systems that incorporate a redox reaction to produce or utilize electrical energy. Almost every car producer has a fuel cell car prototype but still there are several obstacles to overcome before mass production of. 11 Basis of a Fuel Cell.
Fuel cell electrochemistry pdf A fuel cell is a device that converts a fuel such as hydrogen alcohol gasoline or methane into electricity directly. The reactions carried out electrochemically can be energy efficient and less polluting. Click Get Book button to download or read books you can choose FREE Trial service.
Attach the black and one red wire to the fan. Experiments Modeling and Simulations A dissertation submitted to the SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZÜRICH for the degree of Doctor of Technical Sciences presented by Anja Bieberle Dipl-Ing. Batteries Fuel Cells and Supercapacitors is an excellent introductory text to electrochemical energy devices which covers material considerations historical developments of the technology and future prospects spanning fundamental mechanisms to engineering challenges at.
Its operation is based on the electrochemical reactions happening simultaneously on the anode. Electrochemistry MCQ With Answers Pdf Fuel Cells for AIIMS NEET JEE provides a comprehensive coverage of the fundamental concepts in the fields of Electrochemistry. HySA Systems Who we are HySA Systems is a Systems Integration and Technology Validation Competence Centre on HFCT established in 2007 hosted at the University of the Western Cape UWC and.
Conclusions Electrochemical Power Sources. Cell Definition Galvanic cell Galvanic cellflv Chemical energy electrical energy Fuel cell battery Electrolytic cell Electrolysis Splitting Water Animationflv Electrical energy chemical energy Corrosion electroplating Luigi Galvani 9 September 1737. Fuel cells are electrochemical devices that directly convert chemical energy to electrical energy.
We cannot guarantee that Electrochemical Fuel Cells book is available. Technological innovations based on redox reactions date back. Attach the other red wire to two red sockets on the front and back sides of the anode.
Born on October 18 1971 Germany accepted on the recommendation of Prof. Batteries and fuel cells convert chemical energy into electrical energy and are used on a large scale in various instruments and devices. Therefore study of electrochemistry is important for creating new technologies that are.
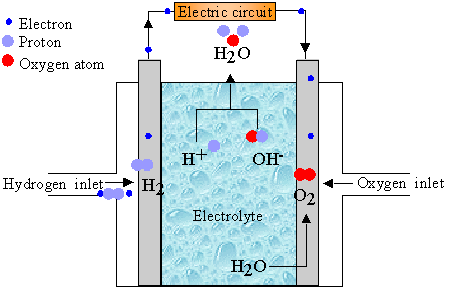
They consist of an electrolyte medium sandwiched between two electrodes Fig. And Rint the internal resistance 216 J. Fuel Cell Electrochemistry A fuel cell is an electrochemical energy converter.
Electrochemistry for Fuel Cells and Electrolysers Professor Bruno G. Fuel cell determining the physicochemical and thermomechanical properties of materials used in the cell components ¾The operating temperature also plays an important role in dictating the degree of fuel processing required. As pointed out in Section 32 electrochemical energy converters principally utilize the chemical energy of fuels with a higher efficiency than heat engines.
Electrochemistry C wwwom FCJJ 34 - Salt Water Fuel Cell Science Kit Lab HIGH SCHOOL Chemistry TEACHER GUIDE 6. Write down anything you observe in the Observations section below. II - Electrochemistry of Fuel Cell - Kouichi Takizawa Encyclopedia of Life Support SystemsEOLSS where E is the standard electromotive force fo r this cell and is expressed as E EO2 EH2.
Isolated oxidation and reduction processes are not much good. UNESCO EOLSS SAMPLE CHAPTERS ENERGY CARRIERS AND CONVERSION SYSTEMS Vol. Therefore study of electrochemistry is important for creating new technologies that are.
Electrochemical fuel cells. 13969 The Electrochemistry of Solid Oxide Fuel Cell Anodes. This should start the fan running.
The consequence of this statement is that fuel cells can contribute. Electrochemistry-Construction of Solar Cells and Fuel Cells Redox reactions reactions involving electron transfer are fundamentally important to chemistry and have been studied by chemists for many years. Combustion Versus Electrochemistry Reactants fuel and oxygen are separated by an electrolyte allowing one reactant and no electrons to pass Conventional method for using fuels - burn them.
Department of Energy Office of Fossil Energy Federal Energy Technology Center. Batteries and fuel cells convert chemical energy into electrical energy and are used on a large scale in various instruments and devices. Request PDF 3.
A fuel cell is a galvanic cell in which the chemical energy contained in a readily available fuel oxidant system is converted directly into electrical energy by means of electrochemical processes in which fuel is oxidized at the anode and oxidant is reduced at cathode while fuel and oxidant are continuously and separately supplied to their. Journal of Power Sources 158 2006 213224 of the fuel cell.

Electrochemistry Notes Chemistry Notes Electrochemistry Chemistry Lessons

Fuel Cells Department Of Chemical Engineering And Biotechnology

Comments
Post a Comment